Pfizer MATISSE Trial Targets Pregnant Women for Bivalent RSV Vaccine
Unprecedented Pregnancy Study Fails on Efficacy, Durability, and Offers No Assurances on Long-Term Safety
All Global Research articles can be read in 51 languages by activating the Translate Website button below the author’s name.
To receive Global Research’s Daily Newsletter (selected articles), click here.
Click the share button above to email/forward this article to your friends and colleagues. Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.
***
Vaccine ideology or hubris appears everywhere! Never had we heard so much about vaccines in our day to day life as laypersons or as healthcare professionals. This certainly is the case for respiratory syncytial virus (RSV) which is largely an infantile problem easily treated with nebulizers when it occurs. Now Pfizer is aggressively testing their investigational bivalent RSV prefusion protein vaccine which contains stabilized preF glycoproteins from the two main cocirculating antigenic subgroups (RSV A and RSV B). Is this really needed? Should pregnant women be put at risk even before product launch for the general childhood population? We can see now that emboldened Pfizer is breaking new ground with clinical trials in late term pregnancy.
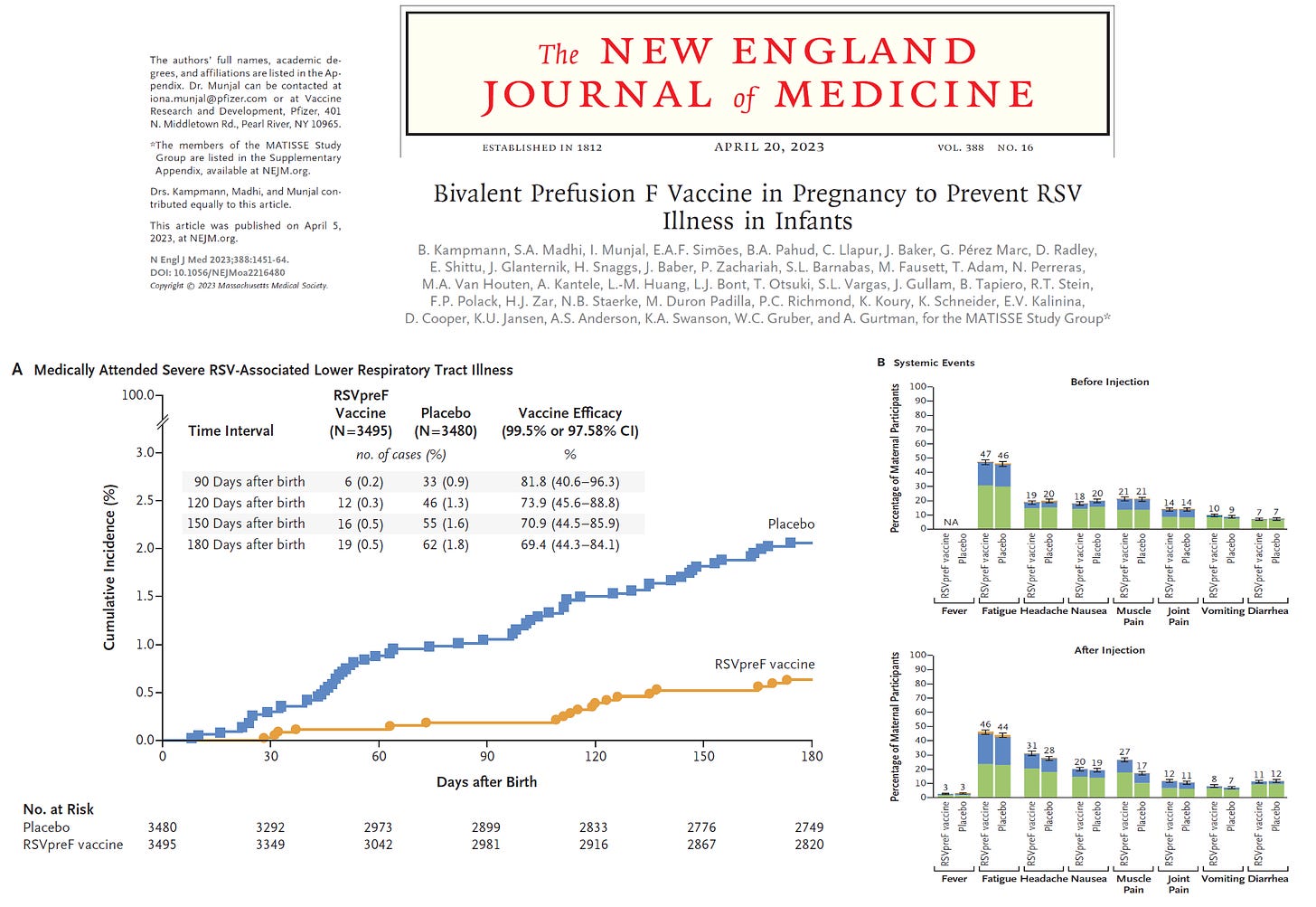
The MATISSE Study group conducted a phase 3, double-blind trial where they randomly assigned, in a 1:1 ratio, pregnant women at 24 through 36 weeks’ gestation to receive a single intramuscular injection of 120 μg of a bivalent RSV prefusion F protein–based (RSVpreF) vaccine or placebo. The main primary efficacy end point occurred in <2% at all time points and was defined as medically attended severe RSV-associated lower respiratory tract illness within 90, 120, 150, and 180 days after birth. A lower boundary of the confidence interval for vaccine efficacy (99.5% confidence interval [CI] at 90 days; 97.58% CI at later intervals) greater than 20% was considered to meet the success criterion for vaccine efficacy with respect to the primary end point. This is far lower than a reasonable acceptance limit of 50% for the lower bound. As you can see at all times, the real effect size could have been lower than 50%. When outcomes are sparse, the point of central tendency is a statistical blur, hence we must rely on the bounds of the confidence limits. Additionally, protection waned rapidly after birth and was not demonstrated to last 12 months.
Kampmann B, Madhi SA, Munjal I, Simões EAF, Pahud BA, Llapur C, Baker J, Pérez Marc G, Radley D, Shittu E, Glanternik J, Snaggs H, Baber J, Zachariah P, Barnabas SL, Fausett M, Adam T, Perreras N, Van Houten MA, Kantele A, Huang LM, Bont LJ, Otsuki T, Vargas SL, Gullam J, Tapiero B, Stein RT, Polack FP, Zar HJ, Staerke NB, Duron Padilla M, Richmond PC, Koury K, Schneider K, Kalinina EV, Cooper D, Jansen KU, Anderson AS, Swanson KA, Gruber WC, Gurtman A; MATISSE Study Group. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N Engl J Med. 2023 Apr 20;388(16):1451-1464. doi: 10.1056/NEJMoa2216480. Epub 2023 Apr 5. PMID: 37018474.
In summary, Pfizer has aggressively advanced RCTs into the pregnant population with no assurances on long term outcomes. Furthermore, they moved the goal posts to make it easer to have a successful trial. We should demand long-term safety, high efficacy (>50% VE as lower bound of CI), and at least one year of durability, for such a rare and easy-to-treat condition.
*
Note to readers: Please click the share button above. Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.
Source
Featured image is from Children’s Health Defense

