More Than 1.3 Million Adverse Events Following COVID Vaccines Reported to VAERS, CDC Data Show

All Global Research articles can be read in 51 languages by activating the “Translate Website” drop down menu on the top banner of our home page (Desktop version).
To receive Global Research’s Daily Newsletter (selected articles), click here.
Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.
***
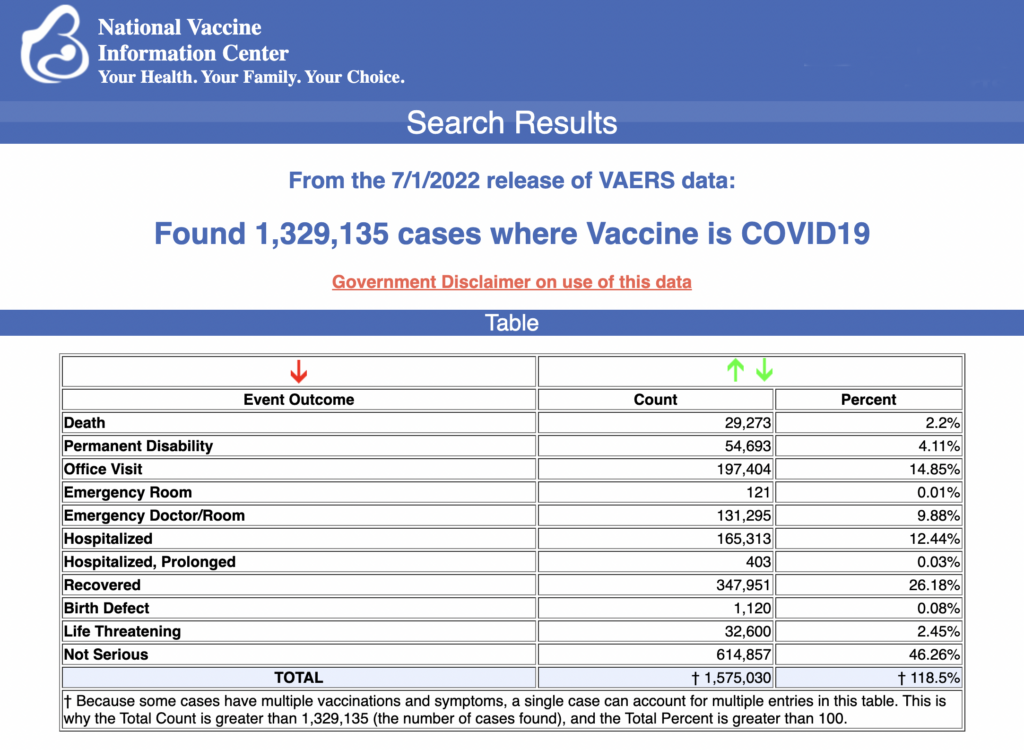
VAERS data released Friday by the Centers for Disease Control and Prevention show 1,329,135 reports of adverse events from all age groups following COVID-19 vaccines, including 29,273 deaths and 241,910 serious injuries between Dec. 14, 2020, and July 1, 2022.
The Centers for Disease Control and Prevention (CDC) today released new data showing a total of 1,329,135 reports of adverse events following COVID-19 vaccines were submitted between Dec. 14, 2020, and July 1, 2022, to the Vaccine Adverse Event Reporting System (VAERS). That’s an increase of 14,541 adverse events over the previous week.
VAERS is the primary government-funded system for reporting adverse vaccine reactions in the U.S.
The data included a total of 29,273 reports of deaths — an increase of 111 over the previous week — and 241,910 serious injuries, including deaths, during the same time period — up 684 compared with the previous week.
Of the 29,273 reported deaths, 18,937 cases are attributed to Pfizer’s COVID-19 vaccine, 7,724 cases to Moderna and 2,545 cases to Johnson & Johnson (J&J).
Excluding “foreign reports” to VAERS, 839,450 adverse events, including 13,547 deaths and 85,321 serious injuries, were reported in the U.S. between Dec. 14, 2020, and July 1, 2022.
Foreign reports are reports foreign subsidiaries send to U.S. vaccine manufacturers. Under U.S. Food and Drug Administration (FDA) regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and does not appear on the product’s labeling, the manufacturer is required to submit the report to VAERS.
Of the 13,547 U.S. deaths reported as of July 1, 15% occurred within 24 hours of vaccination, 19% occurred within 48 hours of vaccination and 58% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 596 million COVID-19 vaccine doses had been administered as of June 29, including 352 million doses of Pfizer, 225 million doses of Moderna and 19 million doses of Johnson & Johnson (J&J).
Every Friday, VAERS publishes vaccine injury reports received as of a specified date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed.
Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
U.S. VAERS data from Dec. 14, 2020, to July 1, 2022, for 6-month-olds to 5-year-olds show:
- 1,941 adverse events, including 64 cases rated as serious and 3 reported deaths.
- 4 reports of myocarditis and pericarditis (heart inflammation).
The CDC uses a narrowed case definition of “myocarditis,” which excludes cases of cardiac arrest, ischemic strokes and deaths due to heart problems that occur before one has the chance to go to the emergency department. - 13 reports of blood clotting disorders.
U.S. VAERS data from Dec. 14, 2020, to July 1, 2022, for 5- to 11-year-olds show:
- 11,638 adverse events, including 300 rated as serious and 7 reported deaths.
- 23 reports of myocarditis and pericarditis.
- 46 reports of blood clotting disorders.
U.S. VAERS data from Dec. 14, 2020, to July 1, 2022, for 12- to 17-year-olds show:
- 32,543 adverse events, including 1,843 rated as serious and 44 reported deaths.
- 62 reports of anaphylaxis among 12- to 17-year-olds where the reaction was life-threatening, required treatment or resulted in death — with 97% of cases attributed to Pfizer’s vaccine.
- 655 reports of myocarditis and pericarditis with 643 cases attributed to Pfizer’s vaccine.
There was one less case reported attributed to Pfizer’s vaccine since the previous week. - 166 reports of blood clotting disorders with all cases attributed to Pfizer. VAERS reported 167 cases of blood clotting disorders in the 12- to 17-year-old age group last week.
- 20 cases of postural orthostatic tachycardia syndrome (POTS) with all cases attributed to Pfizer’s vaccine.
U.S. VAERS data from Dec. 14, 2020, to July 1, 2022, for all age groups combined, show:
- 20% of deaths were related to cardiac disorders.
- 54% of those who died were male, 41% were female and the remaining death reports did not include the gender of the deceased.
- The average age of death was 73.
- As of July 1, 5,629 pregnant women reported adverse events related to COVID-19 vaccines, including 1,761 reports of miscarriage or premature birth.
- Of the 3,623 cases of Bell’s Palsy reported, 51% were attributed to Pfizer vaccinations, 40% to Moderna and 8% to J&J.
- 895 reports of Guillain-Barré syndrome, with 42% of cases attributed to Pfizer, 30% to Moderna and 27% to J&J.
- 2,285 reports of anaphylaxis where the reaction was life-threatening, required treatment or resulted in death.
- 1,733 reports of myocardial infarction.
- 14,171 reports of blood-clotting disorders in the U.S. Of those, 6,334 reports were attributed to Pfizer, 5,088 reports to Moderna and 2,713 reports to J&J.
- 4,251 cases of myocarditis and pericarditis with 2,606 cases attributed to Pfizer, 1,443 cases to Moderna and 187 cases to J&J.
- 13 cases of Creutzfeldt-Jakob disease with 7 cases attributed Pfizer, 5 cases to Moderna and 1 case to J&J.
- 268 cases of POTS with 165 cases attributed to Pfizer, 84 cases to Moderna and 17 cases to J&J.
Uruguay halts COVID vaccine for kids under 13, judge demands government officials turn over Pfizer contracts
Uruguay suspended COVID-19 vaccines for children under 13 after a judge on Thursday issued an injunction halting vaccinations in that age group until government officials turn over contracts with vaccine manufacturers.
Uruguayan government officials and Pfizer were ordered on Wednesday to appear in court after a judge gave them 48 hours to present detailed information on Pfizer’s COVID-19 vaccine while the court considered an injunction request to halt COVID-19.
The government said a confidentiality clause in the contract prevents it from producing the documents, The Washington Post reported.
Judge Alejandro Recarey of the Administrative Litigation Tribunal used his inquisitorial powers to demand the Uruguayan Ministry of Public Health, State Health Services Administration and the President’s Office submit all information regarding the contracts for the purchase of COVID-19 vaccines, including contractual information related to any clauses of civil indemnity or criminal impunity of the suppliers in the event of adverse effects.
The judge also posed a series of questions to government officials and Pfizer regarding the chemical composition, efficacy and safety of COVID-19 vaccines, and required Pfizer to state whether it has “admitted, in any area, internal or external to it and its partners, the verification of adverse effects” of its COVID-19 vaccines in children.
Paul Offit says ‘fix was in’ before FDA panel voted to reformulate boosters
In a June 6 interview with ZDoggMD, vaccine expert Dr. Paul Offit said the FDA’s vaccine advisory panel’s recent meeting on whether to modify COVID-19 boosters was unusual and he felt the panel was led to “vote yes” to reformulate boosters without critical data.
Offit, director of the Vaccine Education Center and professor of pediatrics in the Division of Infectious Diseases at Children’s Hospital of Philadelphia, said:
“I’ve seen nothing like this. I guess the thing that’s most upsetting to me is normally when you get something from the FDA when we have these meetings, you usually get it a few days before you meet. You usually get a couple of hundred pages.
“Here on the other hand, normally you get the EUA [Emergency Use Authorization] submission from the company, which is 85 to 100 pages long, and then you get the FDA’s review of all those data. It’s a very thorough review. Not here though. Here, it was 22 pages from the FDA, which included a half-page on Pfizer’s data and a half-page on Moderna’s data.”
“You could get that from the press release,” Offit said, adding he didn’t see the benefits of reformulated boosters and was surprised that out of 21 voting members, 19 voted “yes.”
Offit also said it was “unusual” that someone from the World Health Organization presented their opinion during the meeting that a modified booster shot was a good idea and the FDA presented their opinion before the advisory committee rendered its advice.
Offit said he believed reformulating COVID-19 boosters was “something that was desired by the Biden administration,” who announced the day after the meeting they had purchased at least 105 million doses from Pfizer with up to 300 million doses.”
Children’s Health Defense asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.
76 doctors urge UK government officials not to authorize COVID vaccines for small children
In a letter to the Medical and Healthcare products Regulatory Agency (MHRA) and other U.K. government officials, 76 doctors explain why the recent U.S. Food and Drug Administration’s (FDA) decision authorizing COVID vaccinations for infants and young children must not happen in the U.K.
The doctors urged the MHRA to consider “very carefully” the move to vaccinate ever younger children against SARS-CoV-2, despite the gradual but significant reducing virulence of successive variants, the increasing evidence of rapidly waning vaccine efficacy, increasing concerns over long-term vaccine harms and the knowledge that the vast majority of the younger age group have already been exposed to SARS-CoV-2 repeatedly and have demonstrably effective immunity.
The doctors said using the risk-benefit analysis that supported the rollout of mRNA vaccines to the elderly and vulnerable in 2021 is totally inappropriate for small children in 2022, and strongly challenged the addition of COVID-19 vaccination into the routine child immunization program despite no demonstrated clinical need, known and unknown risks and the fact that these vaccines still have only conditional marketing authorization.
60,000 unvaccinated guard and reserve soldiers cut from training and pay
About 60,000 Army National Guard members and Army Reserve soldiers who refused to comply with a Department of Defense COVID-19 vaccine mandate are no longer allowed to participate in their military duties and were cut off from some of their pay and benefits, Army officials announced July 1.
Of the more than 40,000 members of the Guard who remain unvaccinated, 14,000 have said they do not intend to ever receive a COVID-19 vaccine, Guard officials told CBS News. Approximately 22,000 Reserve soldiers have refused to get vaccinated.
Soldiers will be allowed to come on duty and earn their pay if it’s for the purpose of getting vaccinated or to take part in separation procedures. If the soldiers continue to refuse to get vaccinated, the consequences could be even more severe.
To date, only six Guard soldiers across all states and territories have received medical exemptions out of 53 who submitted requests, according to Army data. No Reserve soldiers have received a medical exemption.
No Guard or Reserve soldiers have been approved for a religious exemption despite nearly 3,000 requests.
*
Note to readers: Please click the share buttons above or below. Follow us on Instagram and Twitter and subscribe to our Telegram Channel. Feel free to repost and share widely Global Research articles.
Megan Redshaw is a staff attorney for Children’s Health Defense and a reporter for The Defender.
Featured image is from CHD

