The Toxic Impacts of GMO Maize: Scientific Journal Bows to Monsanto, Retracts anti-Monsanto Study

There exist rigid criteria for a serious scientific journal to accept a peer-reviewed paper and to publish it. As well there are strict criteria by which such an article can be withdrawn after publication.
The once-respected Elsevier Journal of Food and Chemical Toxicology has apparently decided to violate those procedures and has announced it is retracting a long-term study on the toxic effects of Monsanto Genetically Modified Organisms (GMOs)—GMO Maize–it published a year ago.
The bizarre announcement comes only six months after Elsevier created a special new position, Associate Editor for Biotechnology (i.e. GMO), and fills it with a former Monsanto employee who worked for Monsanto’s front-organization—the International Life Sciences Institute—which develops industry-friendly risk assessment methods for GM foods and chemical food contaminants and inserts them into government regulations. Sound like something wrong with this picture?
Some Background
In its November, 2012 issue, The Journal of Food and Chemical Toxicology published a paper titled, “Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize.” by Gilles-Eric Séralini and his team of researchers at France’s Caen University.[1] It was a highly important study as it was the first and, astonishingly, still the only long-term study under controlled conditions of possible effects of a diet of GMO Maize treated with Monsanto Roundup herbicide.
Seralini submitted his study results to the respected journal following a rigorous four month review by scientific peers regarding methodology and such. Seralini’s group tested more than 200 rats of a diet of GMO corn over a period of a full two years at a cost of €3 million. The study was done in absolute secrecy to avoid industry pressure.
The publication created an atomic blast rocking the entire edifice of the GMO industry. Pictures of test rats with grotesque cancer tumors appeared in newspapers around the world.
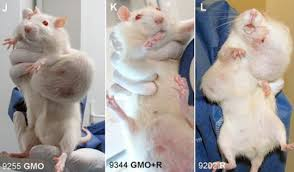
Seralini’s group studied the effect of a Monsanto GMO maize diet on the rats for much longer than Monsanto had in their study submitted to the EU European Food Safety Authority for approval. They did their study for the full two year average life-time instead of just 90 days in the Monsanto study. The long time span proved critical. The first tumors only appeared 4 to7 months into the study. In industry’s earlier 90-day study on the same GMO maize Monsanto NK603, signs of toxicity were seen, but were dismissed as “not biologically meaningful” by industry and EFSA alike. [2]
It seems they were indeed very biologically meaningful.
The study was also done with the highest number of rats ever measured in a standard GMO diet study. They tested “also for the first time 3 doses (rather than two in the usual 90 day long protocols) of the Roundup-tolerant NK603 GMO maize alone, the GMO maize treated with Roundup, and Roundup alone at very low environmentally relevant doses starting below the range of levels permitted by regulatory authorities in drinking water and in GM feed.” [3]
Their findings were more than alarming. Mammary tumors that developed in rats fed GMO corn and/or low levels of Roundup. From the paper “Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize,” published in Food and Chemical Toxicology
The Seralini study concluded, “In females, all treated groups died 2–3 times more than controls, and more rapidly. This difference was visible in 3 male groups fed GMOs. All results were hormone and sex dependent, and the pathological profiles were comparable. Females developed large mammary tumors almost always more often than and before controls; the pituitary was the second most disabled organ; the sex hormonal balance was modified by GMO and Roundup treatments. In treated males, liver congestions and necrosis were 2.5–5.5 times higher. This pathology was confirmed by optic and transmission electron microscopy. Marked and severe kidney nephropathies were also generally 1.3–2.3 greater. Males presented 4 times more large palpable tumors than controls…” [4]
Monsanto on defensive
Monsanto and the related GMO industry immediately went on a war footing to control the potentially fatal damage from the Seralini study. Suddenly, with worldwide attention to the new Seralini results, the EU Commission and its EFSA was under fire as never in their history. How they reacted was worthy of a bad copy of an Agatha Christie murder novel. They piously announced that they had passed the Seralini study on to their EFSA scientific panel for evaluation.
The Brussels EU scientific food regulatory organization, EFSA, was under the gun from the damning results of the long-term Seralini study. EFSA had recommended approval of Monsanto’s NK603 Roundup-tolerant maize in 2009 without first conducting any independent testing. They admitted that they relied on “information supplied by the applicant (Monsanto).” EFSA also admitted that the Monsanto tests on rats were for only 90 days. Seralini’s group noted that the massive toxic effects and deaths of GMO-fed rats took place well after 90 days, a reason why longer-term studied were obviously warranted. [5]
The EFSA concluded at the time of its initial Monsanto NK603 approval in 2009 that, “data provided [by Monsanto-w.e.] are sufficient and do not raise a safety concern.” The Brussels body added, “The EFSA GMO Panel is of the opinion that maize NK603 is as safe as conventional maize. Maize NK603 and derived products are unlikely to have any adverse effect on human and animal health in the context of the intended uses.” [6] Oops!
Now comes this guy Seralini and puts EFSA and the entire regulatory control process for GMO under grave doubt.
The EU Commission was on record stating that no independent non-GMO industry long-term studies were needed on animals to test their safety. The EU guidelines for testing stated, “Toxicological assessments on test animals are not explicitly required for the approval of a new food in the EU or the US. Independent experts have decided that in some cases, chemical analyses of the food’s makeup are enough to indicate that the new GMO is substantially equivalent to its traditional counterpart…In recent years, biotech companies have tested their transgenic products (maize, soy, tomato) before introducing them to the market on several different animals over the course of up to 90 days. Negative effects have not yet been observed.” [7]
The “up to 90 days” is the key statement. Seralini’s study only observed serious tumors and other effects after 120 days in their two-year study.
EFSA Coverup
On November 28, 2012, only a few weeks after the study was published, EFSA in Brussels issued a press release with the following conclusion: “Serious defects in the design and methodology of a paper by Séralini et al. mean it does not meet acceptable scientific standards and there is no need to re-examine (sic!) previous safety evaluations of genetically modified maize NK603.” Per Bergman, who led EFSA’s work, said: “EFSA’s analysis has shown that deficiencies in the Séralini et al. paper mean it is of insufficient scientific quality for risk assessment. We believe the completion of this evaluation process has brought clarity to the issue.” [8]
EFSA argued that Seralini had used the wrong kind of rats, not enough rats and that the statistical analysis was inadequate. By these standards, all toxicity studies on glyphosate and GMOs should be retracted because they used the same type and approximate number of rats as those in the Séralini study.
At the very minimum, the “precautionary principle” in instances involving even the potential for grave damage to the human population would mandate that the EU Commission and its EFSA should order immediate further serious, independent long-term studies to prove or disprove the results of the Seralini tests. Refusal to re-examine its earlier decision to approve Monsanto GMO maize, no matter what flaws might or might not have been in the Seralini study, suggested the EFSA was trying to cover for the GMO agrichemical lobby at the very least.
Many members of the EFSA GMO review panel had documented ties to Monsanto and the GMO industry, a conflict of interest to put it mildly. Corporate Europe Observer, an independent EU corporate watchdog group noted about the EFSA response, “EFSA failed to properly and transparently appoint a panel of scientists beyond any suspicion of conflict of interests; and it failed to appreciate that meeting with Europe’s largest biotech industry lobby group to discuss GMO risk assessment guidelines in the very middle of a EU review undermines its credibility.” [9]
New blood at Elsevier
While the official EFSA statement seemed to take pressure off Monsanto, it clearly was not enough so long as the Elsevier journal study could circulate and be cited around the world.
Then, out of the blue, in May 2013, six months after the Seralini study release, Elsevier announced that it had created a new position, “Associate Editor for Biotechnology.” The person they hired to fill it was Richard E. Goodman, a former Monsanto employee who in addition was with the Monsanto pro-GMO lobby organization, the International Life Sciences Institute (ILSI) which develops industry-friendly risk assessment methods for GM foods and chemical food contaminants and inserts them into government regulations.
As one critical scientific website posed the obvious ethical sham of hiring Monsanto people to control GMO publications, “Does Monsanto now effectively decide which papers on biotechnology are published in FCT? And is this part of an attempt by Monsanto and the life science industry to seize control of science?”[10]
Then on November 24, 2013, six months after Goodman took control of GMO issues at the Journal, Dr A. Wallace Hayes, the editor of the journal Food and Chemical Toxicology decided to retract the study by the team of Professor Séralini.
The reasons for the extraordinary retraction a full year after publishing are in violation of the guidelines for retractions in scientific publishing set out by the Committee on Publication Ethics (COPE), of which FCT is a member. According to the guidelines, the only grounds for a journal to retract a paper are:
• Clear evidence that the findings are unreliable due to misconduct (eg data fabrication) or honest error;
• Plagiarism or redundant publication;
• Unethical research.
Séralini’s paper meets none of these criteria and Hayes admits as much. In his letter informing Prof Séralini of his decision, Hayes concedes that examination of Prof Séralini’s raw data showed “no evidence of fraud or intentional misrepresentation of the data” and nothing “incorrect” about the data, and that the retraction was solely based on the “inconclusive” nature of the findings on tumours and mortality.[11]
As Claire Robinson of GM Watch points out, “inconclusiveness of findings is not a valid ground for retraction. Numerous published scientific papers contain inconclusive findings, which are often mixed in with findings that can be presented with more certainty. It is for future researchers to build on the findings and refine scientific understanding of any uncertainties.” [12]
Elsevier, the publisher of the journal Food and Chemical Toxicology, is one of the giants in worldwide scientific publications. And they are apparently not so rigorous when it comes to making money over scientific principle. In 2009, Elsevier invented an entire medical journal, complete with editorial board, in order to publish papers promoting the products of the pharmaceutical manufacturer Merck. Merck provided the papers, Elsevier published them, and doctors read them, unaware that the “Australasian Journal of Bone and Joint Medicine” was simply a PR vehicle for the drug giant Merck. [13]
Notes
[1] Séralini, G.-E., et al., Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Food Chem. Toxicol. (2012), http://dx.doi.org/10.1016/j.fct.2012.08.005.
[3] Seralini et al., Op. Cit.
[5] European Food Safety Authority (EFSA), Scientific Opinion of the Panel on Genetically Modified Organisms on applications (EFSA-GMO-NL-2005-22 and EFSA-GMO-RX-NK603) for the placing on the market of the genetically modified glyphosate tolerant maize NK603 for cultivation, food and feed uses and import and processing, and for renewal of the authorisation of maize NK603 as existing product, The EFSA Journal (2009) 1137, 1-50.
[7] GMO-Kompass, Food Safety Evaluation–Evaluating Safety: A Major Undertaking, February 15, 2006, accessed in http://www.gmo-compass.org/eng/safety/human_health/41.evaluation_safety_gm_food_major_undertaking.html
[8] EFSA, Séralini et al. study conclusions not supported by data, says EU risk assessment community, EFSA Press Release, 28 November 2012, accessed in http://www.efsa.europa.eu/en/press/news/121128.htm
[9] Corporate Europe Observatory, Op. Cit.
[10] Claire Robinson and Jonathan Latham, PhD, The Goodman Affair: Monsanto Targets the Heart of Science,
May 20, 2013, accessed in http://www.independentsciencenews.org/science-media/the-goodman-affair-monsanto-targets-the-heart-of-science/
[11] Claire Robinson, Journal retraction of Séralini study is illicit unscientific and unethical, GMWatch,, 27 November 2013, accessed in http://www.gmwatch.org/index.php/news/archive/2013/15184-journal-retraction-of-seralini-study-is-illicit-unscientific-and-unethical.

